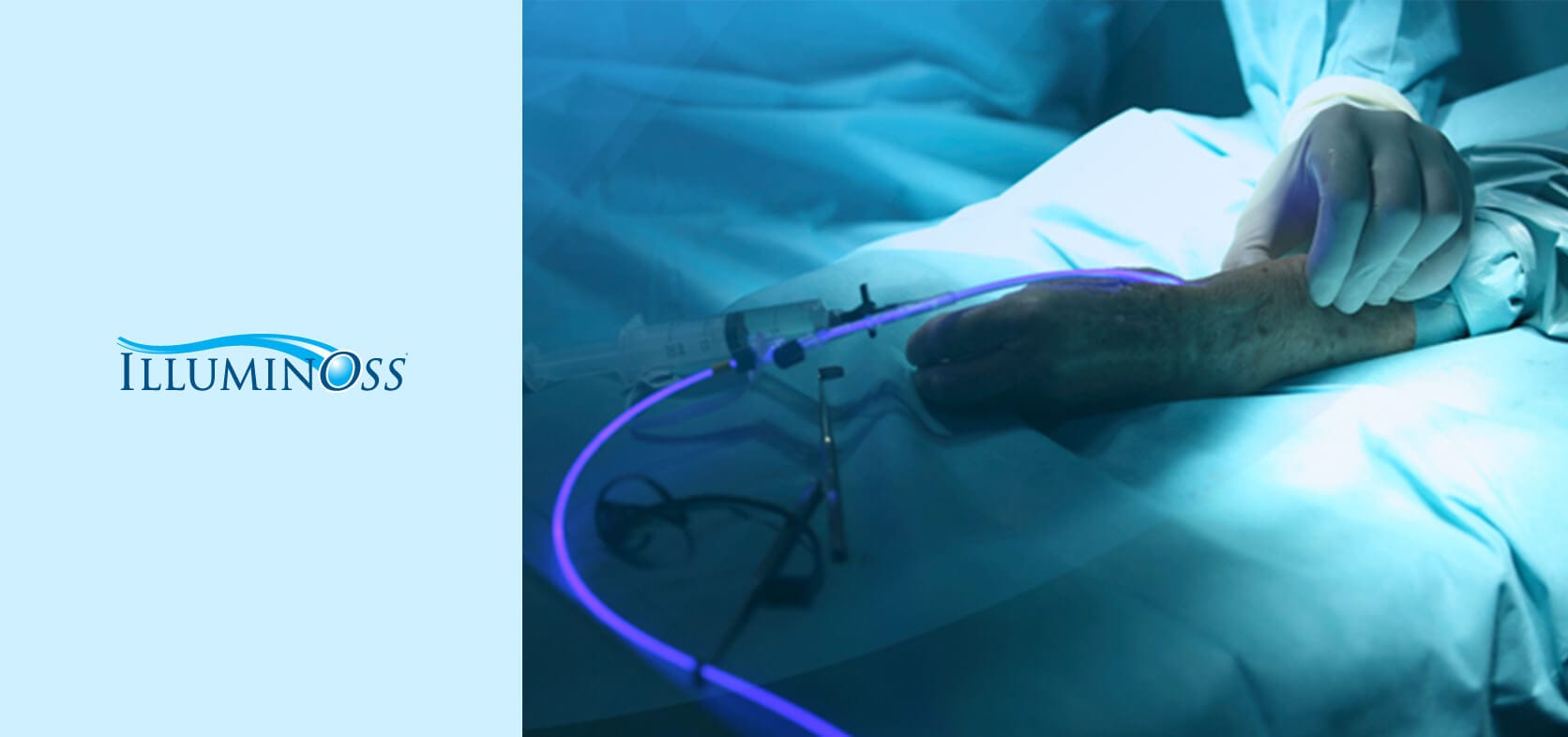


IlluminOss Medical has developed a platform, disruptive technology for the orthopedic trauma market. It has achieved CE Mark approval in Europe, and has filed for expanded indications. IlluminOss is approved in the USA for the treatment of impending and actual pathologic fractures of the humerus, radius, and ulna from metastatic bone disease in skeletally mature patients. The technology centers around minimally invasive patient conforming orthopedic implants that leverage its proprietary bone stabilization technology, the “IlluminOss System”. Through the unique and proprietary combination of balloons, light activated monomers and flexible catheters, IlluminOss has created the world’s first patient conforming intramedullary implant.
 Go back to Portfolio Page
Go back to Portfolio Page